
Author:
Sankalp Lal
Technical Marketing Team Lead
The push from governments around the globe for an electric mode of transport has increased the momentum of manufacturing and sales of electric vehicles. I can confirm this not just from the data in articles but also by observing the increased number of battery electric vehicles (BEVs) and hybrid electric vehicles (HEVs) I spot during the 2 km commute between my home and office. This rise in BEV sales began to shed light on battery fire incidents, which led to safety concerns. Although these incidents are rare, the risks to the public made the study of a battery pack’s thermal efficiency and management crucial for manufacturers.
Testing multiple battery pack designs and configurations to improve efficiency without compromising on safety means manufacturers are spending large sums of money, especially to study thermal runaway. To ease the financial burden, Renault Group took advantage of computational fluid dynamics (CFD) simulations in CONVERGE to predict the propagation of thermal runaway, allowing them to reduce the overall design cost of their battery packs.
One of the biggest challenges in predicting thermal runaway is determining the values of the chemical kinetics/reaction parameters used in thermal runaway reaction mechanisms. These coefficients vary depending on the cell’s chemistry, size, and shape. Predicting thermal runaway without knowing these values would be impossible.
Edwin H. Land, a scientist best known as the co-founder of the Polaroid Corporation, once said, “Any problem can be solved using the materials in the room.” Literally speaking, this may not be true every time. But, it very well held true for the engineers at Renault Group. In collaboration with Convergent Science, they used CONVERGE to take on this challenge.
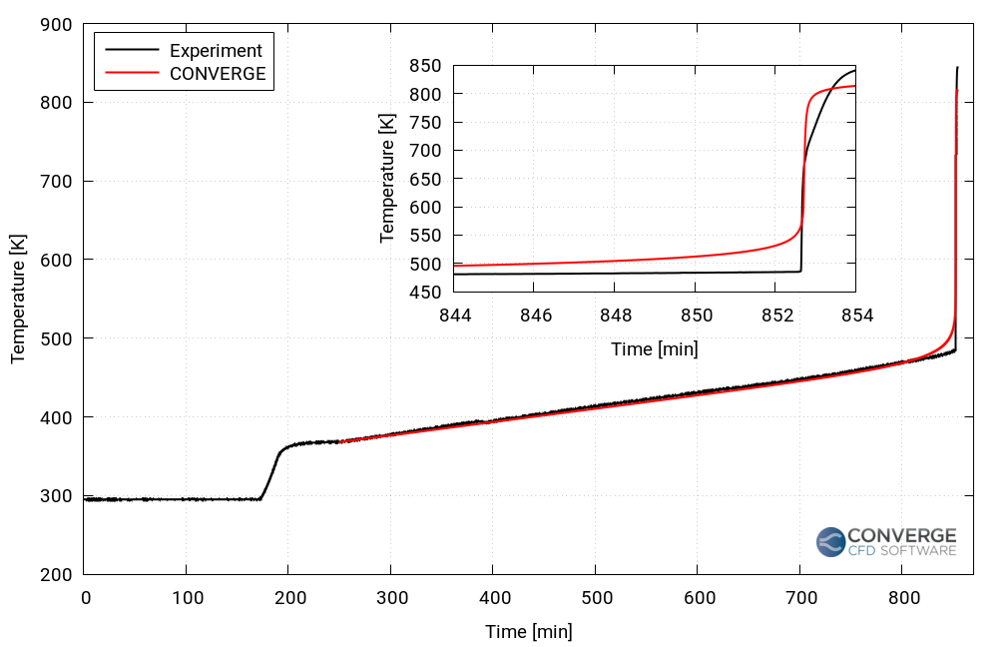
Renault Group performed an in-house experiment in which they heated and forced a single lithium nickel manganese cobalt (Li-NMC) cell to undergo thermal runaway. In particular, they noted the thermal runaway timing and temperature. They then prepared a case setup in CONVERGE with conditions defined similarly to the experiment. Simulating the experimental setup allowed them to compare the results and calibrate the thermal runaway model by optimizing the reaction parameters until the timing and temperature matched.
The results from the simulation matched well with the experiment (shown in Figure 1). CONVERGE was able to accurately capture the time and temperature beyond which thermal runaway occurs in the cell. The calibrated Arrhenius coefficients were thus fed to the battery pack simulation setup.
The engineers at Renault and Convergent Science constructed a 16-cell battery pack stacked in series (Figure 2) using the previously discussed Li-NMC cell. The aim was to predict and analyze the propagation of thermal runaway after its initiation in one of the cells (8th cell) in the pack. Groups of four cells each were separated by compression pads (Figure 3). Thermal resistances between the cells and the directional differences in thermal conductivity in the battery pack were defined via the contact resistance and anisotropic conductivity features in CONVERGE, respectively. The calibrated Arrhenius coefficients from the single-cell simulation above were supplied to the thermal runaway model in CONVERGE to predict the thermal runaway propagation.
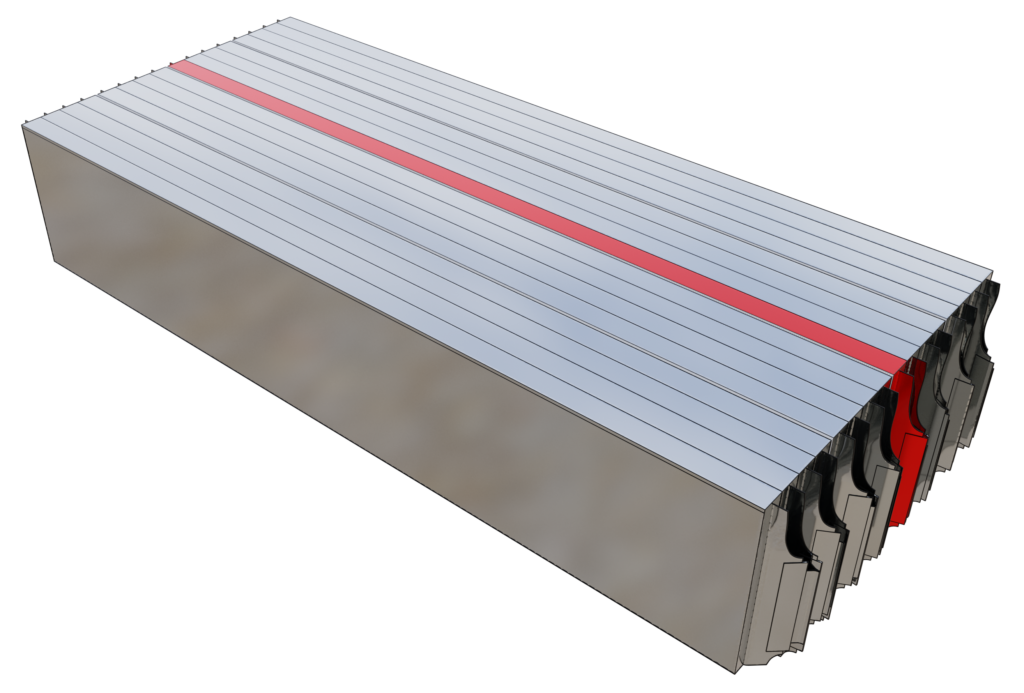
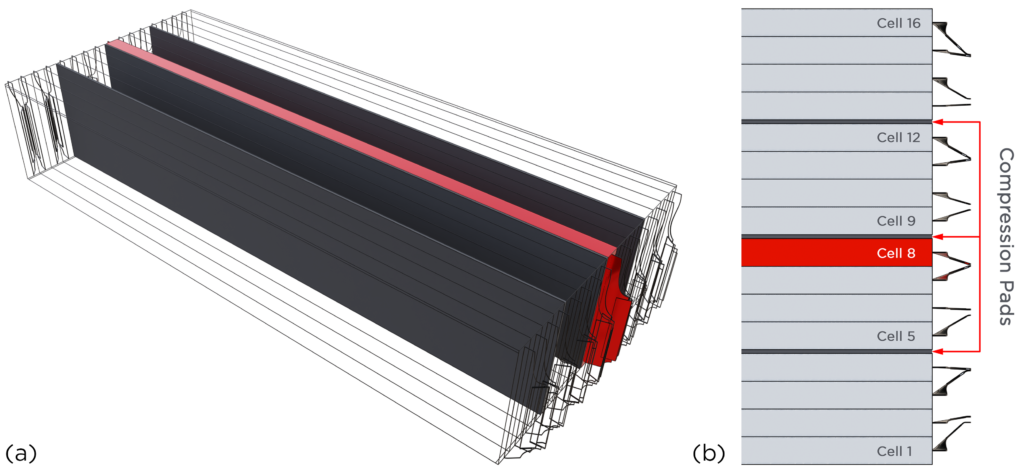
It was assumed that every cell in the battery pack was similar in size, shape, and composition. In reality, there are minor differences in the cells that will affect the thermal runaway timing and temperature.
By intuition, we can gauge that the temperature penetration in cells 5 to 7 will be higher compared to cells 9 to 12. But, what about the maximum temperature in each of the neighboring cells? Will those cells become hot enough to lead to the propagation of thermal runaway? Let’s take a look at CONVERGE’s prediction in the following section.
The temperature distribution in the battery pack was studied for ~200 seconds after the initiation of thermal runaway. Video 1 shows the temperature penetration in the neighboring cells. The lower penetration from cells 8 to 9 is due to the presence of the compression pad in between which offers a higher thermal resistance. The absence of a compression pad between cells 7 and 8 results in more penetration in that direction.
Figure 4 shows a plot of the maximum temperature in the cells in the vicinity of cell 8. The temperature rise was the highest in cell 7, attained in the first few seconds after the initiation of thermal runaway. Cell 6 reached its peak temperature about 50 seconds after the thermal runaway, and the temperature rise in cells 10 and 11 is much less compared to the other cells.
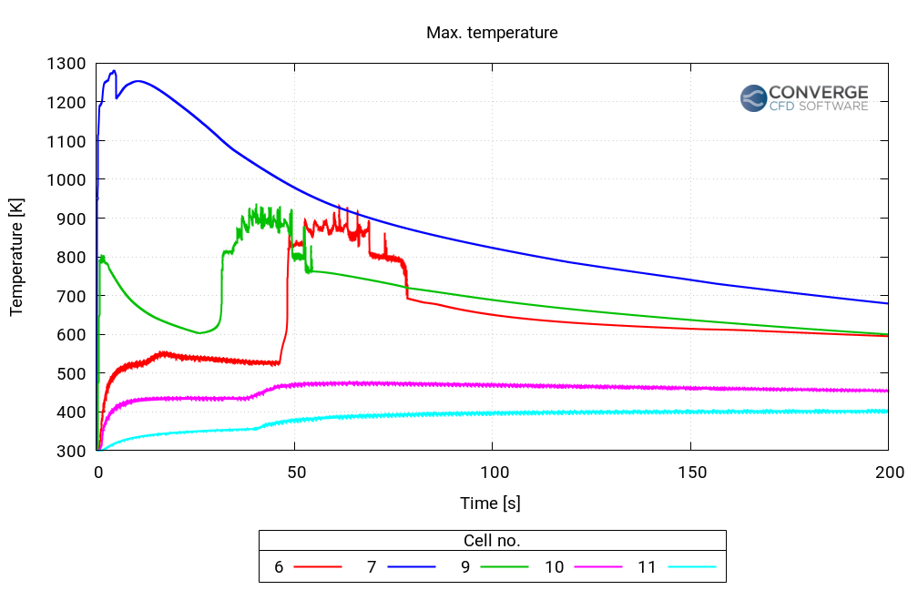
Figure 5 summarizes the complete workflow to predict thermal runaway and its propagation in a battery pack. Using the approach developed in CONVERGE, Renault Group was able to predict and study the thermal runaway event in their pouch cell battery pack. In the future, this model can be used to further study venting and its effects on the neighboring cells in the battery pack.
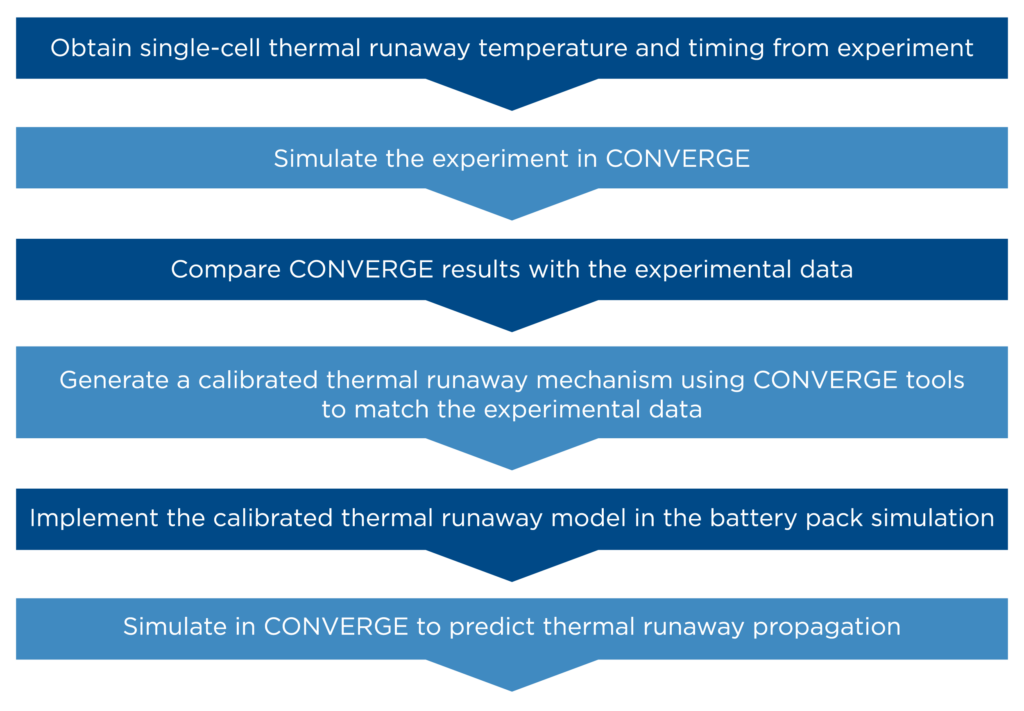
While this approach requires multiple steps, following this workflow enables users to perform detailed simulations of battery packs to obtain more reliable and predictive results. In addition to the useful features described in this article, CONVERGE is equipped with a detailed chemistry solver to accurately solve the thermal runaway reaction chemistry and determine the heat source, and autonomous meshing to automatically generate and refine the mesh during runtime. This suite of features empowers CONVERGE users to efficiently and accurately predict thermal runaway propagation, making it a viable and cost-effective solution.
We would like to express our sincere gratitude to Renault Group for permitting us to use their studies in this blog. Their contributions have been essential in creating this informative content.
DESIGNING SAFER BATTERIES THROUGH SIMULATION
A LESS EXPENSIVE METHOD FOR PREDICTING THERMAL RUNAWAY PROPAGATION


